- Project
- Expected result
- Duration
- Grant
- Memscap AS
- SC Euromedica SRL
The Medicare project is divided into Reseach and Development, Investments and Traning activities. Design, production and qualification of a new molding tools, membrane tools, blister packaging, transducer solutions for medical disposable product.
Purchase of molding machines and polymer materials for testing and qualification. Process development of all disposable parts. Increase production in assembly lines for medical domes, infusion linesets and dialysis parts. Establish and qualify new sterilization facility in Romania to reduce cost of sterilization and transport.
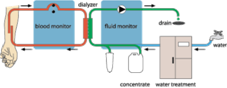
Establish a state-of-the art production line for disposable articles for medical use at SC Euromedica SRL.
Establish new sustainable production of all Memscap disposable medical devices in Euromedica, Iasi, Romania, consequently reduce production cost per unit, reduce transport cost and logistic challenges in order to increase the production volume.

Planned Project duration March 2009 - April 2011



Grant amount awarded: € 1.860.000
Grant rate: 60%

MEMSCAP AS (Norway) is a fully owned subsidiary of the French MEMSCAP S.A. The business is focused on sensor- and transducer solutions, based on micro-electro-mechanical systems (MEMS) technology, for the Medical and the Aerospace industry. MEMSCAP AS has a unique background in the Norwegian MEMS environment. The history of the company goes back to the early sixties were its initial technology platform were developed at SINTEF, Oslo. Through different foundations of MEMS companies, the business was a part of the Norwegian SensoNor ASA until 2002. MEMSCAP AS has today 65 employees with a revenue of 10 MEURO for the year 2007.
MEMSCAP AS has been operating in the Medical and Aerospace industry for more than 30 years. High-end micromachined silicon based Medical sensors and transducers have been designed and manufactured in the facility in Norway. Most important is the AE800 flow sensor for Respirators, breathing support systems and anaesthesia machines for the entire Medical world market. More than 2 million sensors have been delivered to the Medical market since the introduction in the mid 1960’s.
The AE840 and SP844 Invasive blood pressure transducer, Memscap have supplied famous brands like Siemens Medical, Philips, Hewlett Packard, Dräger Medical, Datex and General Electric during the last decades. The disposable domes and line sets are currently produced several places in Europe, Norway, Denmark, Sweden, Germany, Holland and Romania. A lot of saving can be done to collect the production in one location in Romania.
In Aerospace segment, Memscap delivers pressure sensors in application areas like: Air Data Computers, Carbine Pressure and Engine Control. The customer base is concentrated both in the US- and the European Aerospace industry. The present versions of the piezoresistive pressure sensor has obtained design-in approval for a variety of aircrafts with the following reference list: Boeing, SIKORSKY, Eurofighter 2000, McDonnell Douglas MD90, Airbus, Cessna, McDonnell Douglas DC9, Harrier/AV8B, Bell 412, Dassault, Lockheed, JSF and others.
EuroMedica is a privately owned company established in 1998 in the manufacturing of single use medical devices. The factory is located in a new building, where on a total area of 1500 m2 there are three class 8 clean rooms and the appropriate storage spaces. EuroMedica has currently a total of 30 trained employees committed to quality.
EuroMedica’s Quality Management System has demonstrated compliance to the requirements of ISO 9001:2000 (excluding clause 7.3) and ISO 13488:2003 and therefore has been certified for the assembly, marketing and distribution of medical non-active devices by Nemko Certification – part of IQ Net (certificates # NO-900677 and NO-908010).
The company currently manufactures under contract for some European companies, according to customer specification, a large range of products such as: Intravenous products (drip sets, flow control systems, extension lines, y set etc.) multi – lumen manometry catheters for clinical investigations, urinary catheters and bags. EuroMedica also packs dental devices, surgical eye swabs, surgical kits, etc. The products are individually packed or in bulk. Some products are supplied sterile, some non-sterile as per customer specification.
EuroMedica is a privately owned company established in 1998 in the manufacturing of single use medical devices. The factory is located in a new building, where on a total area of 1500 m2 there are three class 8 clean rooms and the appropriate storage spaces. EuroMedica has currently a total of 30 trained employees committed to quality.
EuroMedica’s Quality Management System has demonstrated compliance to the requirements of ISO 9001:2000 (excluding clause 7.3) and ISO 13488:2003 and therefore has been certified for the assembly, marketing and distribution of medical non-active devices by Nemko Certification – part of IQ Net (certificates # NO-900677 and NO-908010).
The company currently manufactures under contract for some European companies, according to customer specification, a large range of products such as: Intravenous products (drip sets, flow control systems, extension lines, y set etc.) multi – lumen manometry catheters for clinical investigations, urinary catheters and bags. EuroMedica also packs dental devices, surgical eye swabs, surgical kits, etc. The products are individually packed or in bulk. Some products are supplied sterile, some non-sterile as per customer specification.


